Europe style for Low Molecular Heparin - Heparin Sodium (Porcine Source) – CSBIO
Europe style for Low Molecular Heparin - Heparin Sodium (Porcine Source) – CSBIO Detail:
INDICATIONS:
Prevention and treatment of thrombosis or thrombotic diseases (such as myocardial infarction, thrombophlebitis, pulmonary embolism and so on); also used in the treatments of disseminated intravascular coagulation (DIC) derived from all kinds of reasons; hemodialysis, extra-corporeal circulation, catheterization, microvascular surgery and anticoagulation treatment of some blood samples and instruments.
COMPANY ADVANTAGE
1.full product Chains:
We have the full product Chains, which starts from the Porcine mucosa with be processed to Crude heparin, Heparin sodium API that could be depolymerized to the API of Enoxaparin sodium, Dalteaprin sodum and Nadroparin calcium. We could control the product from the starting material and make sure the tracebility which is required by the regulated market. At present, we have our own crude heparin workshop, which could help us to not only control the cost bust also assure the quality of our product.
2.APIs’ Production Lines:
Facilities and Equipment Systems
There are dedicated production workshops respectively for Heparin API, Enoxaparin Sodium API and Dalteparin Sodium&Nadroparin Calcium API. These three workshops are all established and have their respective dedicated production equipments, HVAC system and purify water system, which could avoid the cross contamination.
3.Product qualification
For Heparin Sodium API, we have passed the followings audits, US-FDA, EDQM, CFDA, Germany/Korean and Turkey authority, CEP, EIR-LETTER, China and Germany GMP are available.
4.Production capacity is enough:Heparin Sodium API: 5 million mega
5.We basically estsabilshed GMP Six-system that can comply with the standard of EU GMP,US FDA CGMP and Chinese GMP which could make sure our product comply with the international quality systems.
6.The company is located in the Zhengding area of China (Hebei) Pilot Free Trade Zone, close to Shijiazhuang Airport and the high-speed railway station, with a good transportation location. On August 26, 2019, the State Council issued the “Overall Plan for China (Hebei) Pilot Free Trade Zone”. The Zhengding area focuses on the development of bio-medicine, international logistics and other industries. We will strive to build the company into a leading enterprise in the domestic heparin industry.
7.Main market: Italy, Russia, Ukraine, Belarus, Indian, Korean, Argentina, Turkey, Iran and so on.
8.Payment: TT in advance
Delivery details: within in 30days after confirmed the order by air
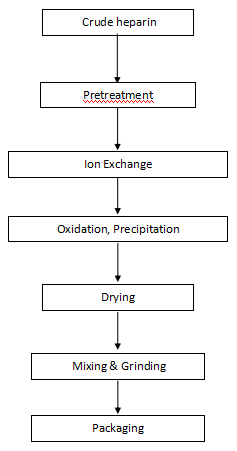
Flow Chart of Heparin Sodium
Products Specification (EP)
|
TESTS |
SPECIFICATIONS |
|
Characters |
White or almost white, hygroscopic powder. |
|
Solubility |
Freely soluble in water |
|
Identification |
A. It complies with the requirements described under Assay |
|
B. The ratio of anti-factor Xa activity to anti-factor IIa activity ranges between 0.9~1.1. |
|
|
C. 1H-NMR Spectrum: comply with the Ph. Eur. specification of heparin about 1H-NMR requirements. |
|
|
D. The principal peak in the chromatogram obtained with test solution is similar in retention time and shape to the principal peak in the chromatogram obtained with reference solution. |
|
|
E. It complies with the test for sodium. |
|
|
Appearance of solution |
The solution is clear and not more intensely coloured than intensity 5 of the range of reference solutions of the most appropriate color. |
|
pH |
5.5 ~ 8.0 |
|
Nucleotide impurities |
Absorbance at 260 nm: ≤0.15 |
|
Protein |
≤0.5% |
|
Related substances |
A. Sum of dermatan sulfate and chondroitin sulfate: not more than the area of the peak due to dermatan sulfate and chondroitin sulfate in the chromatogram obtained with reference solution (e) (2.0 per cent); B. Any other impurity: no peak with an area greater than 0.01 times the area of the peak due to dermatan sulfate and chondroitin sulfate in the chromatogram obtained with reference solution (e) is detected (corresponding to a disregard limit of 0.02 per cent) |
|
Nitrogen |
1.5%~2.5% |
|
Sodium content |
10.5%~13.5% |
|
Loss on drying |
≤8.0% |
|
Bacterial endotoxin |
Less than 0.01 IU/IU of heparin |
|
Potency |
≥180 IU/mg |
Products Specification (USP)
|
TESTS |
SPECIFICATIONS |
|
Characters |
White or almost white, hygroscopic powder. |
|
Solubility |
Freely soluble in water |
|
Identification |
|
|
Appearance of solution |
The solution is clear and not more intensely coloured than intensity 5 of the range of reference solutions of the most appropriate color. |
|
pH |
5.0 ~ 7.5 |
|
Nucleotide impurities |
NMT 0.1% (w/w) is found. |
|
Protein |
NMT 0.1% (w/w) is found. |
|
Related substances |
|
|
Loss on drying |
≤5.0% |
|
Bacterial endotoxin |
It contains NMT 0.03 USP Endotoxin Units/USP Heparin Unit. |
|
Potency |
≥180 IU/mg |

Product detail pictures:

Related Product Guide:
Science, simplified:How vaccines work against COVID-19
Our mission is usually to turn into an innovative provider of high-tech digital and communication devices by providing worth added design and style, world-class producing, and repair capabilities for Europe style for Low Molecular Heparin - Heparin Sodium (Porcine Source) – CSBIO , The product will supply to all over the world, such as: America, Mumbai, Vancouver, We take measure at any price to attain essentially the most up-to-date gear and procedures. The packing of nominated brand is our a further distinguishing feature. The solutions to assure years of trouble-free service has attracted a great deal customers. The goods are obtainable in improved designs and richer variety, they're produced scientifically of purely raw supplies. It accessible in a variety of designs and specifications for the selection. The newest forms are much far better than the previous one and they're extremely popular with several clients.
The sales manager is very patient, we communicated about three days before we decided to cooperate, finally, we are very satisfied with this cooperation!





