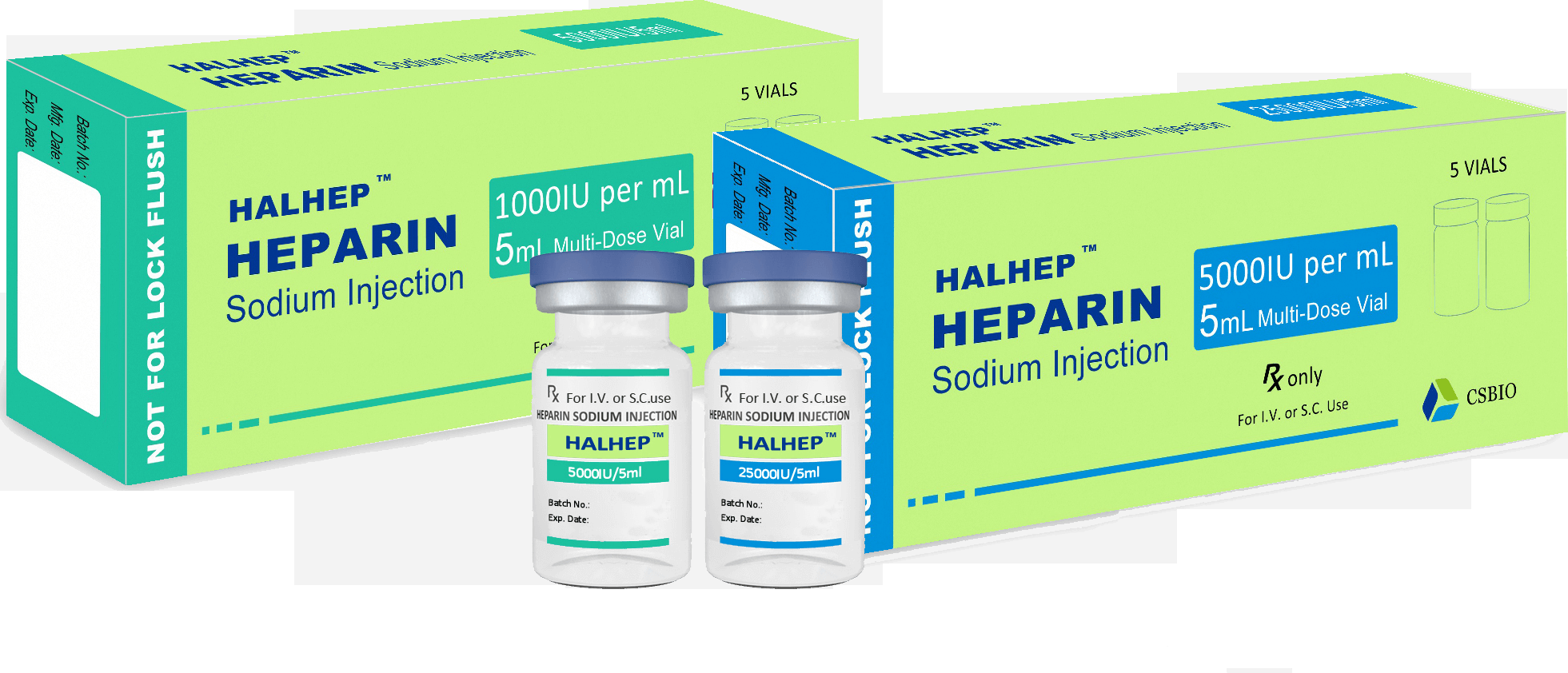
Heparin Sodium Injection(Bovine Source)
INDICATIONS:
Prophylaxis of deep vein thrombosis and pulmonary embolism.
Treatment of deep vein thrombosis and pulmonary embolism, unstable angina pectoris and acute peripheral arterial
occlusion.
Prophylaxis of mural thrombosis following myocardial infarction.
In extracorporeal circulation and haemodialysis.
OVERDOSE:
Bleeding is the chief sign of heparin overdose. When clinical circumstances (bleeding) require reversal of heparinization, protamine sulfate (1% solution) by slow infusion will neutralize Heparin sodium. Each mg of protamine sulfate neutralizes approximately 100 IU of heparin.
STORAGE:
Not higher than 25 °C, can not be frozen
Hebei Changshan Biochemical Pharmaceutical Co., Ltd. was founded in 2000. On 19 August 2011, the company was listed on the Growth Enterprise Market of Shenzhen Stock Exchange (stock code 300255). The company has four production bases, nine subsidiaries, two overseas companies, and two joint ventures. The company is a biochemical pharmaceutical enterprise, integrating research and development, production, sales, and import and export trade. It is a key high-tech enterprise with products from heparin crude products to low molecular weight heparin injections and a complete heparin industrial chain in the field of heparin, and a leading pharmaceutical company in the production of domestic heparin products.
What We Do
Since its inception, the company has been researching, developing, manufacturing and marketing drugs for cardiovascular, cerebrovascular diseases and diabetes and anti-cancer drugs. The company has actively expanded its fields, including medical devices, beauty products, and precision medicine. The products are exported to the United States, Germany, France, Italy, Spain, South Korea, Japan, Russia, India, the Philippines, and other countries in the world.
For many years, CSBIOhas focused on the research and development and registration of heparin series products and hyaluronic acid series products at home and abroad, and will gradually increase its research and development efforts in the fields of diabetes drug abenazin and Antineoplastic drug c-met. We will build a strategic layout with the heparin series as the core and the development of multi-disciplinary innovative drugs.